Products
Cyclic-di-GMP Assay
Documentation
Main Info
- DESCRIPTION Spinach aptamer-based c-di-GMP sensor.
- KIT SIZE Sufficient for 100 assays in 96-well plate formats.
- STORAGE CONDITIONS Stable for 2 year at -20 ºC.
Assay Description
The cyclic-di-guanosine monophosphate (c-di-GMP) assay is a simple mix-and- read, highly selective, HTS-ready assay to measure c-di-GMP levels in cells. This homogenous assay can be used to detect c-di-GMP in any biochemical or enzymatic reaction that produces c-di-GMP or in cell-based applications to monitor intracellular c-di-GMP concentrations.
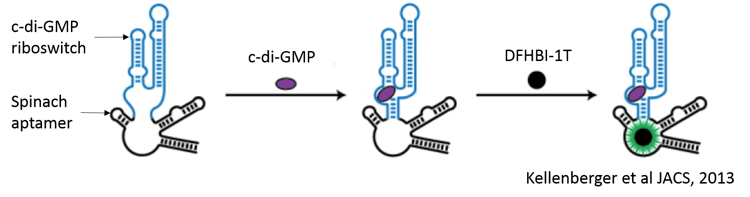
Assay Principle
The evolution of the fluorescent signal is dependent on the initial binding of c-di-GMP to the c-di-GMP riboswitch in the sensor. This results in the stabilization of the SpinachTM aptamer, which in turn binds DFHBI-1T to produce fluorescence. The fluorescence can be measured using GFP/FITC filter sets or with excitation at 482nm and emission at 505nm.
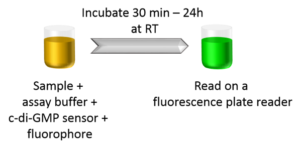
Ease of Use
The c-di-GMP assay is easy-to-use and homogenous. Simply incubate your samples with the provided assay reagents for 30 min and read the samples on a fluorescence plate reader with FITC/GFP filter or excitation wavelength 482nm and emission wavelength 505nm.
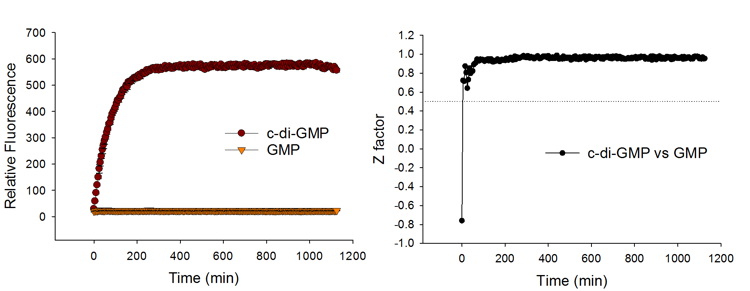
Quick Response and Good Signal Stability
The c-di-GMP sensor produces signals rapidly upon c-di-GMP detection, and thus allows fast measurement. The fluorescent signal is stable over time and thus allows batch-mode processing of samples. Excellent HTS parameters are attained as early as 30 min of assay setup and remain high for a prolonged period of time.
Broad Dynamic Range
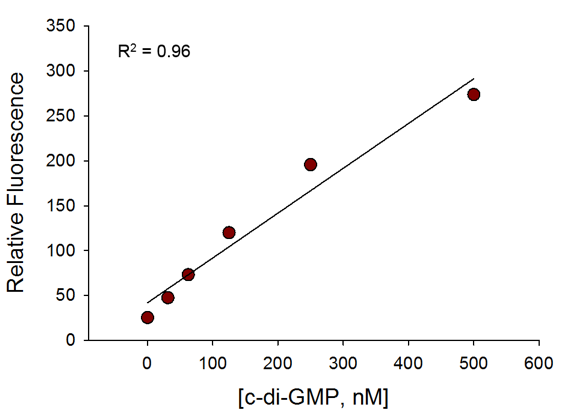
The assay is sensitive even at 50nM of c-di-GMP and has a broad dynamic range.
Selectivity
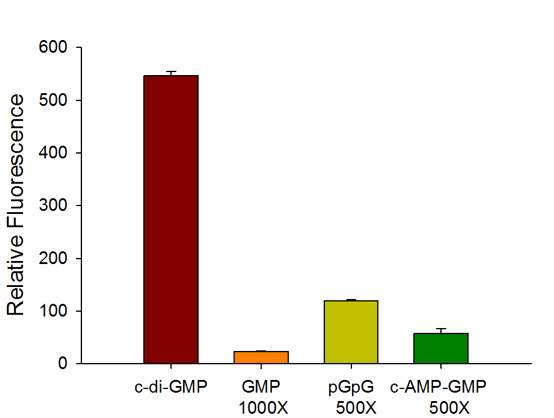
The c-di-GMP sensor is highly selective for c-di-GMP with no interference from common counter ligands.
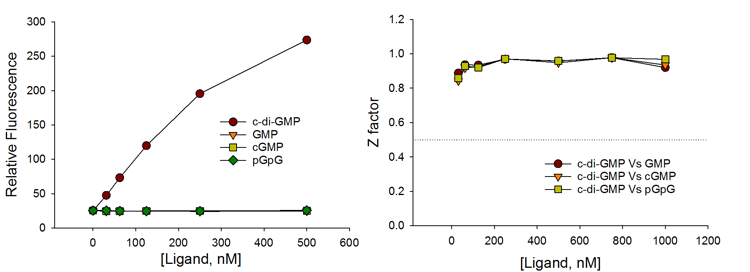
The assay achieves excellent HTS assay parameter (Z factor >0.9) even when compared with 500 – 1,000-fold excess of counter ligands such as GMP, pGpG, and c-AMP-GMP.
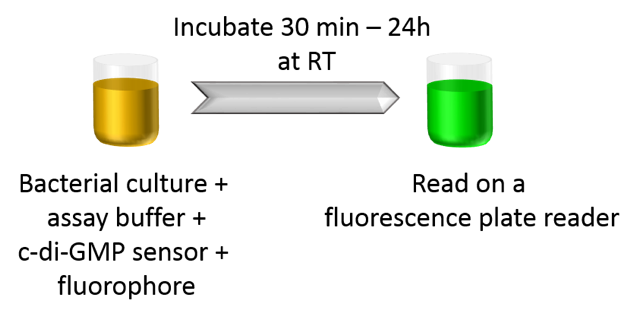
Compatibility with Cell-Based Assays
The c-di-GMP assay can be used to measure intracellular c-di-GMP concentrations in bacteria in a homogenous format without pelleting, lysis, or wash steps. Simply supplement the assay buffer containing bacterial culture with BC reagent, add sensors and fluorophores, incubate for 30 min, and read on a fluorescence reader.
c-di-GMP Estimation in Bacteria
E.coli cells expressing wild type WspR (a diguanylate cyclase from Pseudomonas aeruginosa) were treated with nitrofurazone (antibiotic), dimethyl formamide/DMF (carrier), or left untreated (control). In addition, the bacteria were also plated at varying cell densities to simulate varying c-di-GMP levels. Cyclic-di-GMP concentrations in bacterial cells growing in a 96-well plate were measured without any pelleting, washes, lysis, or organic extraction.
c-di-GMP Standard Curve
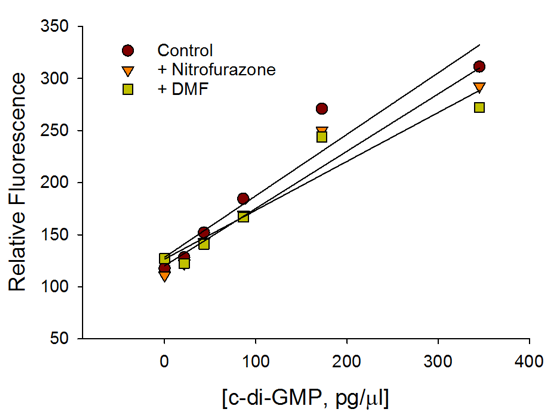
Presence of antibiotic or carrier did not affect the performance of the c-di-GMP assay.
c-di-GMP Estimate in WT WspR Cells
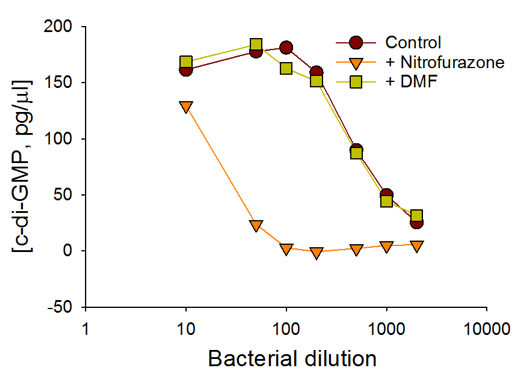
The c-di-GMP assay shows the changes in c-di-GMP concentrations in bacteria in response to antibiotic treatment and varying bacterial cell densities.
This product was cited in:
Condensins are essential for Pseudomonas aeruginosa corneal virulence through their control of lifestyle and virulence programs. Zhao H et al. Mol Microbiol. 2022.
Cyclic-di-GMP signaling links biofilm formation and Mn(II) oxidation in Pseudomonas resinovoran. Piazza A et al. ASM J mBio. 2022.
Vibrio parahaemolyticus and Vibrio vulnificus in vitro biofilm dispersal from microplastics influenced by simulated human environment. Leighton RE et al. Front. Microbiol. 2023.
Superoxide dismutase A (SodA) is a c-di-GMP effector protein governing oxidative stress tolerance in Stenotrophomonas maltophilia. Sun XY et al. Microbiol Res. 2024.
BrfA functions as a bacterial enhancer-binding protein to regulate functional amyloid Fap-dependent biofilm formation in Pseudomonas fluorescens by sensing cyclic diguanosine monophosphate. Guo M et al. Microbiol Res. 2024