Products

DFHBI
Information
3,5-difluoro-4-hydroxybenzylidene imidazolinone (DFHBI)
MOLECULAR FORMULA:
C12H10F2N2O2
MOLECULAR WEIGHT:
252.22
CAS NAME/NUMBER:
4H-Imidazol-4-one, 5-[(3,5-difluoro-4-hydroxyphenyl)methylene]-3,5-dihydro-2,3-dimethyl-, 5 (Z)- / 1241390-29-3
Fluorescence Spectra
Absorption and fluorescence emission spectra of DFHBI in pH 7.4 buffer.
PRESENTATION: Lyophilized dye
QUALITY ASSURANCE: Products are analyzed by 1H NMR and LC-MS and provided at purity of >95% by HPLC.
USAGE STATEMENT: This product is intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals. Due to the highly specific nature of fluorophores and aptamers, we cannot predict or be held responsible with respect to how LucernaTM products will behave in its customers’ systems. Researchers using LucernaTM products should conduct optimization studies to achieve the optimal result possible for their intended application.
Data
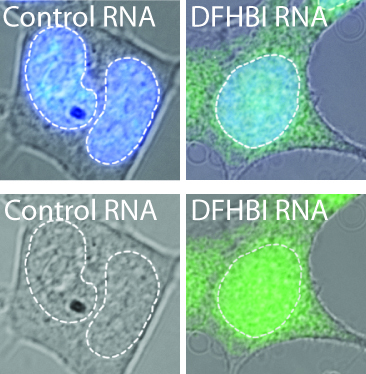
Figure 1. HEK293T cells were transfected with either 5S-control aptamer or 5S-SpinachTM plasmids and incubated with 20 µM DFHBI. Images are phase overlay with Hoechst-stained nuclei (blue) and SpinachTM fluorescence (green).
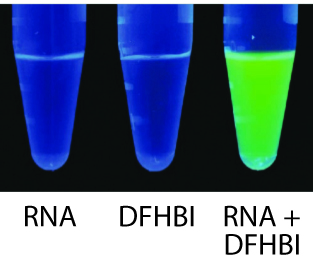
Figure 2. SpinachTM RNA robustly activates the fluorescence of DFHBI. Each tube contained the indicated solution and was irradiated at 465 nm.
Reference
RNA mimics of green fluorescent protein. Paige JS, Wu KY, Jaffrey SR. Science. 2011 Jul 29;333(6042):642-6.
Fluorescence imaging of cellular metabolites with RNA. Paige JS, Nguyen-Duc T, Song W, Jaffrey SR. Science. 2012 Mar 9;335(6073):1194.
This Product Was Cited In
RNA signal amplifier circuit with integrated fluorescence output. Akter F and Yokobayashi Y. ACS Synth Biol. 2015 May 15.
Design, synthesis, and application of Spinach molecular beacons triggered by strand displacement. Bhadra S and Ellington AD. Methods Enzymol. 2015 Jan 15.
Live-cell imaging of mammalian RNAs with Spinach2. Strack RL and Jaffrey SR. Methods Enzymol. 2015 Jan 6.
Monitoring mRNA and protein levels in bulk and in model vesicle-based artifical cells. van Nies P, Canton AS, Nourian z, Danelion C. Methods Enzymol. 2015 Jan 6.
Using Spinach aptamer to correlate mRNA and protein levels in Escherichia coli. Pothoulakis G and Ellis T. Methods Enzymol. 2015 Jan 5.
In vitro analysis of riboswitch-Spinach aptamer fusions as metabolite-sensing fluorescent biosensors. Kellenberger CA and Hammond MC. Methods Enzymol. 2014 Dec 19.
The Spinach RNA Aptamer as a Characterization Tool for Synthetic Biology. Pothoulakis G, Ceroni F, Reeve B, Ellis T. ACS Syn Biol. 2013 Sep 13.
A superfolding Spinach2 reveals the dynamic nature of trinucleotide repeat-containing RNA. Strack RL, Disney MD, Jaffrey SR. Nat Mehtods. 2013 Dec; 10(12):1219-24.
Gene position more strongly influences cell-free protein expression from operons than T7 transcriptional promoter strength. Chizzolini F, Forlin M, Cecchi D, Mansy SS. ACS Syn Biol. 2013 Dec 9.

